Which Statement Describes a Hydrogen Bond Between Two Water Molecules
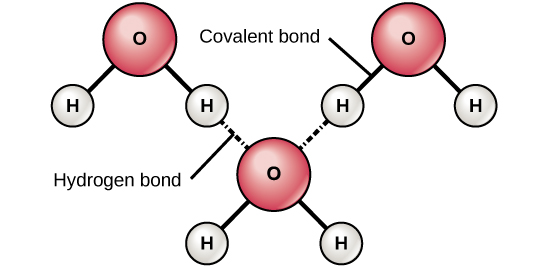
Figure 224 Hydrogen Bonds between Water Molecules. Due to the electronegativity difference between the atom pairs mentioned electrons are unevenly shared across the covalent bond.

Lesson Explainer Hydrogen Bonding Nagwa
- The bond between hydrogen atoms in diatomic hydrogen H2.

. Up to 24 cash back 6. This tiny force of attraction is called a hydrogen bond. Ø Hydrogen bond formed between two molecules is called intramolecular hydrogen bond.
A hydrogen bond is weaker than an ionic bond or a covalent bond but stronger than van der Waals forces. The key to understanding waters chemical behavior is its molecular structure. This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons.
Which statement describes a hydrogen bond between two water molecules. B Oxygen is able to covalently bond with additional hydrogen molecules when the temperature drops and molecular motion slows down. Water molecules show adhesion.
Which statement describes a hydrogen bond between two water molecules. The image above depicts water molecules. Multiple hydrogen bonds occur simultaneously in water because of its bent shape and the presence of two hydrogen atoms per molecule.
Hydrogen bonds play an important role in biochemistry and produce many of the unique properties of water. Covalent bonds within water molecules expand when solid. Nuclear forces form expansive crystals as freezing occurs.
A weak bond in which the oxygen atom of one molecule takes an electron away from the hydrogen atom of another water molecule a weak bond between the slightly negative oxygen atom of one water molecule and the slightly positive hydrogen atoms of another water molecule a weak bond. Which statement describes a hydrogen bond between two water molecules. Ionic bonds between water molecules expand when solid.
Hydrogen bonds between water molecules expand when solid. Proteins are important biological molecules. Hydrogen bonding is an attractive force between two molecules that relies on the slight polarity of the O-H O-F or O-N bond.
A water molecule consists of two atoms of hydrogen linked by covalent bonds to the same atom of oxygen. Hydrogen bonds may form between atoms within a molecule or between two separate molecules. - The bond between oxygen and hydrogen in water H2O - Electrons are not equally shared by the atoms in a covalent bond - One atom of a covalent bond has higher electronegativity than the other atom in the bond Nonpolar bonds.
Water molecules are polar. Consequently the electrons in the water molecule spend slightly more time around the oxygen atomic center and less time around the hydrogen atomic. Ø The bond dissociation energy of covalent bond between O and H in water molecule is 470 kJmol.
-Water is a polar molecule that disrupts ionic bonds between sodium and chloride. Hydrogen donates electrons to oxygen as freezing occurs. A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent.
Water molecules are polar. I Describe the formation of a hydrogen bond between two molecules of water and explain why water can form these bonds. Water molecules have weak bonds.
All Of The Following Statements Help To Explain Why Water Molecules Form Hydrogen Bonds Exceptthe attraction of an atom for the electrons in a covalent bond. The figure below shows how the bent shape and two hydrogen atoms per molecule allows each water molecule to be able to hydrogen bond to two other molecules. Notice that the bonds occur between the weakly positive charge on the hydrogen atoms and the weakly.
Water molecules are large. Atoms of oxygen are electronegative and attract the shared electrons in their covalent bonds. All of the following statements help to explain why water molecules form hydrogen bonds except.
3 ii Hydrogen bonds allow water to act as a solvent. Water is polar due its bent structure and an unequal sharing of electrons the slightly positive hydrogen atom is attracted to the slightly negative oxygen atom within a single water molecule. Which of the following statements describe hydrogen bonding between water molecules or describe properties of water that lead to hydrogen bonding between water molecules.
Hydrogen bonding occurs because the weakly negative oxygen atom in one water molecule is attracted to the weakly positive hydrogen atoms of two other water molecules Figure 224. A weak bond in which the oxygen atom of one molecule takes an electron away from the hydrogen atom of another water molecule a weak bond between the slightly negative oxygen atom of one water molecule and the slightly positive hydrogen atoms of another water molecule a weak bond. -a weak bond between the slightly negative oxygen atom of one water molecule and the slightly positive hydrogen atoms of another water molecule.
This bond is very weak. Hydrogen Bonds - Opposite charges attract one another. Because hydrogen floats the greater the number of hydrogen atoms bound to an oxygen molecule the more buoyant it becomes.
Hydrogen bonds also form between water molecules. Ø The bond dissociation energy of hydrogen bond in liquid water is about 23 kJmol. The slight positive charges on the hydrogen atoms in a water molecule attract the slight negative charges on the oxygen atoms of other water molecules.
Which statement explains why hydrogen bonds are able to form between water molecules. Hydrogen bonds exist causing the water to be less dense. The electrons spend more time around th.
The bond between two oxygen atoms formed FAQwhich statement describes how the bond between two oxygen atoms formed adminSend emailDecember 13 2021 minutes read You are watching which statement describes how the bond between two.

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
Why Life Depends On Water Biology For Non Majors I

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
No comments for "Which Statement Describes a Hydrogen Bond Between Two Water Molecules"
Post a Comment